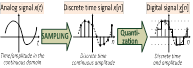|
|
A John Wiley & Sons,
Ltd., Publication |
|||
|
Home |
“True
progress is that which places technology in everyone’s hands” |
|||
|
· Contents · Approach · Target Readers · Pedagogy · Goal 3 · Reviews |
Official Book Website
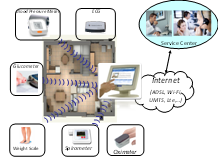
Medical Instrument
Design
and Development Claudio Becchetti, Alessandro Neri, John Wiley & Sons, 590 pages |
|
||
|
|
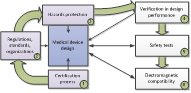
This book explains all of the stages involved in
developing medical devices; from concept to medical approval-certification.
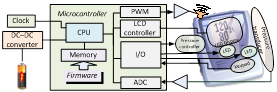
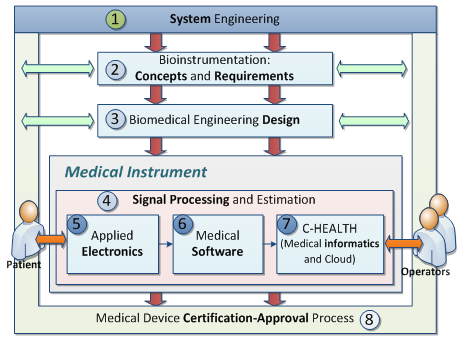
These topics are introduced according to the following chapter organization: system engineering (Ch.1), bioinstrumentation
requirements (Ch.2) and design (Ch.3), signal processing (Ch. 4),
electronics, software (Ch. 6),
medical informatics and e-Health (Ch. 7), medical certification/approval (Ch.
8 ).
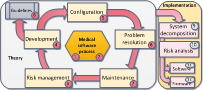
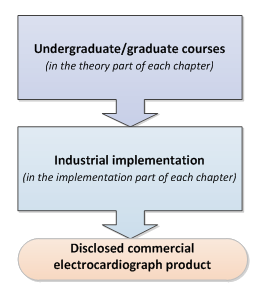
Book structure (Chapter numbers are circled) The book explains how the theory is translated into
industrial medical products. The sequence of
the chapters reflects the product development lifecycle.
Each chapter is
focused on a specific University course and is divided into two sections: theory
and implementation. The theory sections explain the main concepts and
principles which remain valid across technological evolutions of medical
instrumentation. The Implementation sections show how the theory is
translated into a medical product
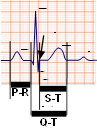
using a market-sold Electrocardiograph (ECG or EKG) disclosed in its design by the
Gamma Cardio Soft manufacturer. The ECG is used as an example as it
is a suitable device to explore and to fully understand medical
instrumentation. It is sufficiently simple but encompasses all the main areas
involved in developing medical electronic equipment. ·
Students of
Biomedical engineering courses (upper-level undergraduate and graduate)
Through this book, we emphasize the system-wide
technical design approach that encompasses the basic theory, the associated
implementation techniques – disclosed for a market product – and
the application of regulations and standards. A good background in mathematics and electronics is helpful for using
this book, but undergraduates will be helped by the various checklists,
recap tables and notes that summarize the main technical background. The book has 250 figures and 145 tables; most of them offering
a schematic representation of the main concepts. Text in bold is used to communicate
concepts in speed reading. Figures have been designed with different graphic
aspects to help readers’ memorization. The implementation part is
organized to offer a didactic perspective, starting from a general medical
device and arriving at the ECG details. Chapters are self-contained, addressing the relevant biomedical
engineering courses. At the same time, chapters are logically linked
as they describe the design process from conception to certification (from Chapter
1 to Chapter 8) with increased technical details. The sections of each chapter are introduced following the sequential
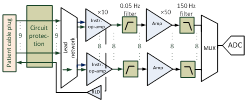
and logical process of the design. For example, in the electronics chapter,
the paragraphs are organized according to the flow of the input signal. Each chapter begins with a conceptual map showing the relations
among the sections and the associated topics. Regulations, standards and
technologies are critical elements of product design. We have addressed the
main concepts and principles of these topics avoiding specific regulations,
standard versions or technological implementations as possible. Regarding references, we have preferred a selected list of
public domain material and tutorials that are helpful to further investigations.
·
“True progress is that which places technology in
everyone’s hands” ·
Through the disclosure of a marketed
product, authors aim to diffuse the medical device and the ECG-related design
know-how. ·
For the ECG, please refer also to the Open Ecg Resources Web Page that contains ECg resources under open licence (
Creative Common, GPL, … ) ·
"The book tackles
a very interesting topic, namely "how medical devices and instruments
are designed from the conception to the market placement". Its utility
and educational potential is high because it shows how the principles of
design and the methodological steps for final product implementation are
applied in a specific case, an electrocardiograph (ECG) device. " - From a
reviewer of the editorial review process. ·
"..., the
electronic details of a market-sold ECG device are fully presented. The
choice for such a device as a case study is fortunate since it is a device
that "is sufficiently simple to be addressed in a book at University level,
but it is also adequate to show the main components required for any complex
medical device design". "- From a reviewer of the editorial review
process. ·
"The succession of
chapters in the book is sound, leading, step-by-step, from general principles
to the final development stages. The division of each chapter into a
theoretical and an implementation part is appropriate and helps greatly in
connecting each step in the design and development phases from both the
theoretical principles and the actual case study perspective. This is
crucial, since the material existing in the book is inevitably overwhelming.
The reader is also helped by the preface of each chapter ("Chapter
Organization"). " The content is very relevant. ...
The use of this text would be beneficial in a 3-4 credit hour
educational course, especially at the graduate level and as a reference in
professional/company engineering situations. I will use this text for Masters
course at my University." - From a reviewer of the editorial review process. ·
" the book seems
well suited for biomedical engineering courses and is also of interest to
design engineers. Its publication will be an interesting and rather
innovative contribution, especially due to its "all-inclusive" scope
in the presentation of design and implementation of medical devices, in
conjunction with the specific details given for the commercial ECG device
that constitutes the case study of the book." - From a reviewer
of the editorial review process. |
|||
|
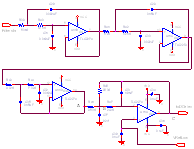
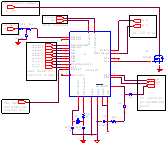
Gamma Cardio CG, the Open Ecg disclosed in the book: ·
A commercial Ecg connected to the PC,
sold in the European market, with performance compliant with Ansi Aami EC 11
standard ·
The hardware is fully licensed
under Creative Common
Attribution Non Commercial Alike 3.0 Unported ·
This product has been certified under the
European Medical Device Directive (93/42/EEC, 2007/47) in class IIb, electrical
isolation class II CF (double insulation, applied parts suitable for direct
cardiac application) (standards EN 60601-1, EN 60601-2-25, EN 60601-1-2). ·
All the hardware circuits and PCB are
disclosed. Software for PC and firmware of the hardware board will follow. · Electrical circuit schematics ·
All the schematics are contained in the
book: "Medical Instrument Design and Development", Becchetti, Neri, Wiley. · PCB There is a bug on PCB (Printed Circuit Board).
Special mention on this site for who discovers the bug first |
||||
|
© Gamma Cardio Soft S.r.l. –
All Right Reserved – gammacardiosoft@gmail.com |
||||
|
|